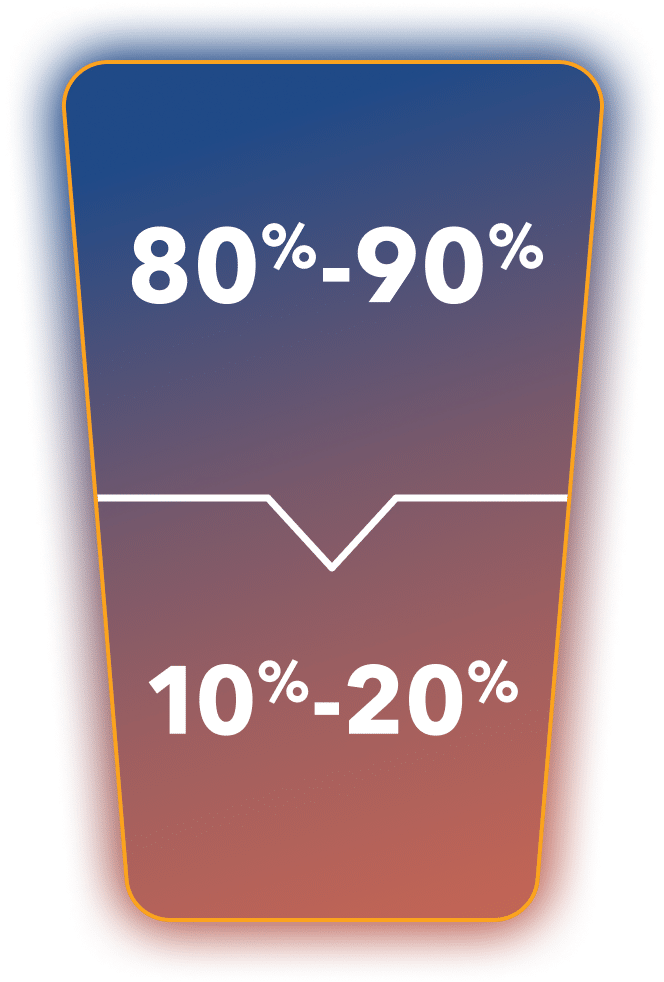
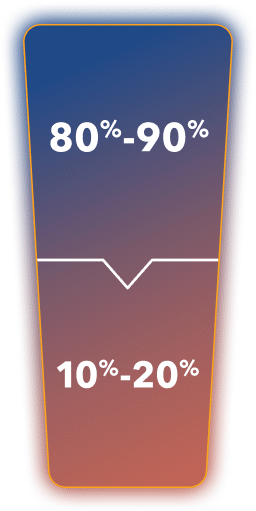
~80%-90% of patients
with prostate cancer
remain castration sensitive within 5 years of follow-up
from diagnosis3*
~10%-20% of patients with prostate cancer progress to castration-resistant disease within 5 years of follow-up3*
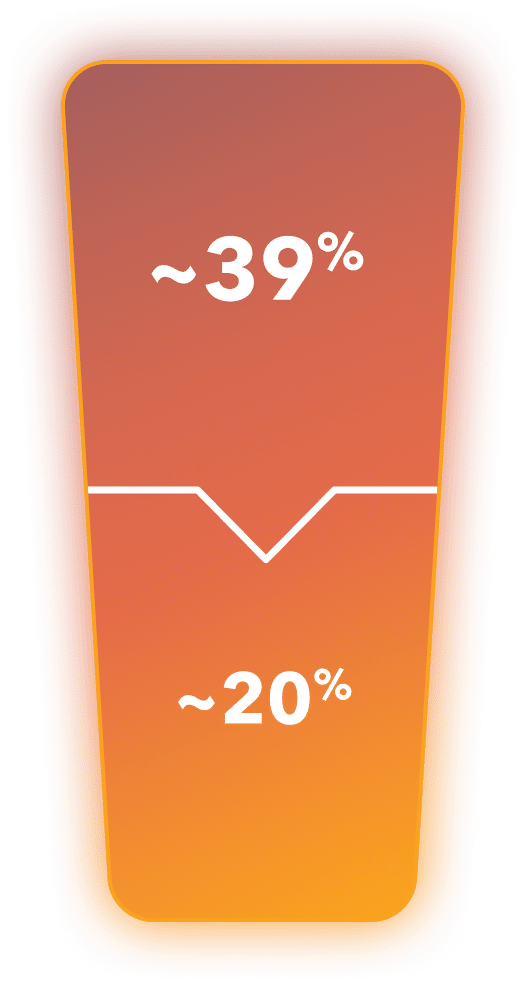
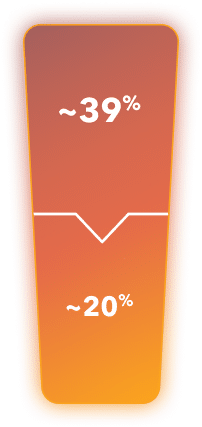
~39% of mCRPC patients in the US are positive for HRR mutations4†
~20% of mCRPC patients in the US are positive for BRCA mutations specifically4†
*Percentages are from an observation based on a systematic review of 5 studies that included 70,086 patients observed for up to 12 years.
†Based on a global survey conducted in 2020 of mCRPC patients with BRCA or other HRR mutations. Percentages and ns shown are from the US population.
Multiple genetic tests are commercially available to help you assess BRCA mutations in patients with prostate cancer.
National Comprehensive Cancer Network® (NCCN®) makes no warranties of any kind whatsoever regarding their content, use, or application and disclaims any responsibility for their application or use in any way.
BRCA, BReast CAncer gene; HRR, homologous recombination repair; mCRPC, metastatic castration-resistant prostate cancer; PARPi, poly (adenosine diphosphate-ribose) polymerase inhibitor.
RUBRACA® (rucaparib) is indicated for the treatment of adult patients with a deleterious BRCA mutation (germline and/
or somatic)-associated metastatic castration-resistant prostate cancer (mCRPC) who have been treated with androgen receptor-directed therapy. Select patients for therapy based on an FDA-approved companion diagnostic for RUBRACA.
The duration of RUBRACA treatment prior to the diagnosis of MDS/AML ranged from < 2 months to approximately 72 months. The cases were typical of secondary MDS/
cancer therapy-related AML; in all cases, patients had received previous platinum-containing chemotherapy regimens and/
or other DNA damaging agents.
In TRITON3, MDS/AML occurred in 2 out of 201 patients (1%) with a BRCA mutation treated with RUBRACA. The duration of therapy with RUBRACA in patients who developed secondary MDS/cancer therapy-related AML varied from 1.4 to 2.3 years.
If MDS/AML is confirmed, discontinue RUBRACA.
Most common adverse reactions of patients with BRCA-mutated mCRPC treated with RUBRACA in TRITON3 (≥10%, Grade 1-4) were fatigue/asthenia (61%), musculoskeletal pain (53%), nausea (51%), decreased appetite (34%), diarrhea (31%), constipation (31%), vomiting (25%), dyspnea (19%), dysgeusia (18%), edema (18%), abdominal pain (17%), dizziness (16%), weight decreased (16%), rash (13%), headache (12%), peripheral neuropathy (12%), photosensitivity reaction (12%), and urinary tract infection (10%).
Most common adverse reactions of patients with BRCA-mutated mCRPC treated with RUBRACA in TRITON2 (≥ 20%; Grade 1-4) were fatigue/asthenia (62%), nausea (52%), decreased appetite (28%), rash (27%), constipation (27%), vomiting (22%), and diarrhea (20%).
Most common laboratory abnormalities of patients with BRCA-mutated mCRPC treated with RUBRACA in TRITON2 (≥ 35%; Grade 1-4) were increased ALT (69%), decreased leukocytes (69%), decreased phosphate (68%), decreased absolute neutrophil count (62%), decreased hemoglobin (59%), increased alkaline phosphatase (44%), increased creatinine (43%), decreased lymphocytes (42%), increased triglycerides (42%), decreased platelets (40%), and decreased sodium (38%).
If concomitant administration with warfarin (a CYP2C9 substrate) cannot be avoided, consider increasing the frequency of international normalized ratio (INR) monitoring.
For medical information inquiries within the U.S., contact pharma& at medinfo.us@pharmaand.com.
You may report adverse events to the FDA at 1-800-FDA-1088 or www.fda.gov/
medwatch.
Alternatively, to report an adverse event or reaction, contact pharma& at pv@pharmaand.com.
To report a product complaint, contact pharma& at complaints@pharmaand.com.
for the treatment of BRCA-mutated mCRPC1
Learn more about the updated indication in the full Prescribing Information
ARPI, androgen receptor pathway inhibitor; BRCA, BReast CAncer gene; mCRPC, metastatic castration-resistant prostate cancer;
PARPi, poly (adenosine diphosphate-ribose) polymerase inhibitor.